 A new review confirms the widespread use of paracetamol by pregnant women and the risk associated with this practice.
A new review confirms the widespread use of paracetamol by pregnant women and the risk associated with this practice.
Using paracetamol during pregnancy may affect fetal development and increase the risks of neurodevelopmental, reproductive and urogenital disorders, a new review study suggests.
According to this new review, paracetamol should only be used by pregnant women when medically indicated, should be used at the lowest effective dose and for the shortest possible time, the report suggests.
Paracetamol (N-acetyl-p-aminophenol (APAP), also known as acetaminophen, is the ingredient of more than 600 medications currently in the market for the treatment of mild to moderate pain and to reduce fever. The drug is the most commonly used over-the-counter analgesics and has been used in Australia since the 1950s, for both adults and children.
Pregnant women also commonly self-medicate with paracetamol, and the drug is widely considered safe. However, according to this new review, more caution is needed from both doctors and regulatory bodies, to ensure this drug doesn’t have adverse effects in women and their offspring.
About the findings
This new review brought together 13 international experts, including clinicians (specialising in neurology, obstetrics and gynaecology, and paediatrics), epidemiologists and basic scientists specialising in toxicology, endocrinology, reproductive medicine and neurodevelopment. The multi-disciplinary team of authors reviewed experimental and epidemiological literature in English available on PubMed published between 1 January 1995 and 25 October 2020. Data regarding respiratory outcomes were excluded from this review due to the known effect of confounding factors.
Among the key findings of this new review, the authors found that paracetamol-based medications are amongst the most commonly used medications globally, used by about 65% of pregnant women, in the USA, and about 50% worldwide. From a health perspective, the study also identified several adverse outcomes associated with use of this drug during pregnancy, including:
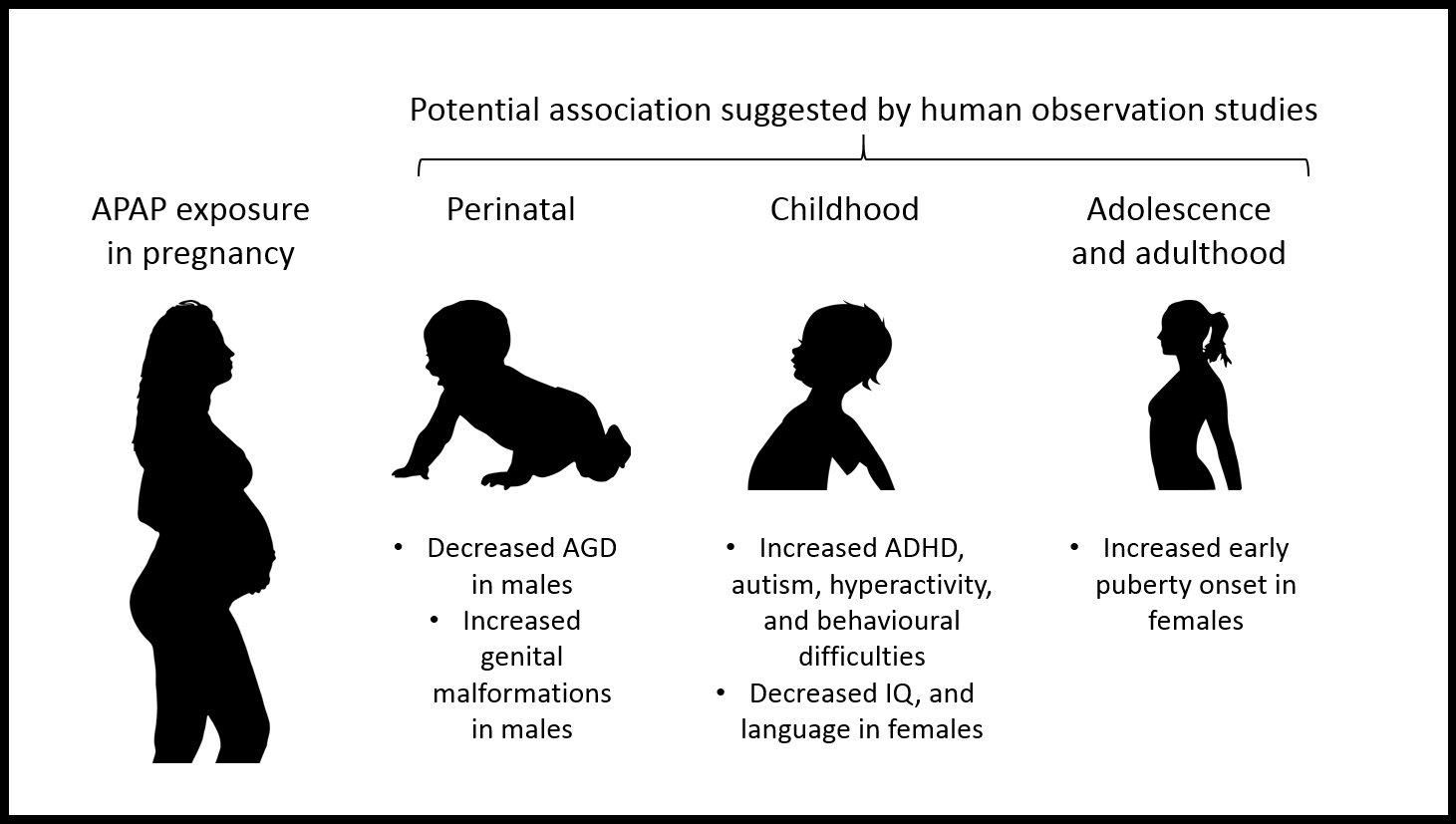
- Reproductive and neurobehavioural abnormalities in offspring of both sexes.
- Earlier female pubertal development.
- Increased the risk of adverse neurodevelopmental and behavioural outcomes, such as attention deficit hyperactivity disorder (ADHD), autism spectrum disorder, language delay (in girls) and decreased intelligence quotient.
According to the authors, these findings strongly suggest that the timing and duration of maternal use of paracetamol-based medications are critical factors to be considered by a doctor.
The authors conclude their study with a note of caution to health care professionals and policy makers, who ought to consider the evidence at hand and make recommendations about the use of paracetamol-based medications by pregnant women. The authors end their review quoting the recent statement made by the Targeting Environmental Neuro-Developmental Risks (TENDR) initiative, which says:
“We as a society should be able to take protective action when scientific evidence indicates a chemical is of concern, and not wait for unequivocal proof that a chemical is causing harm to our children. Evidence of neurodevelopmental toxicity of any type — epidemiological or toxicological or mechanistic — by itself should constitute a signal sufficient to trigger prioritisation and some level of action.”
Paracetamol (also called acetaminophen) should be used only when medically indicated during pregnancy and at the lowest effective dose for the shortest possible time, according to a Consensus Statement published in Nature Reviews Endocrinology. The authors call for a focused research effort to study how paracetamol may affect foetal development and propose a series of precautionary measures that should be taken in the meantime.
Paracetamol is widely used during pregnancy, with estimates suggesting that it is used by up to 65% of those who are pregnant in the United States, and over 50% worldwide. However, an increasing amount of research suggests that prenatal exposure to paracetamol may affect foetal development, which might increase the risk of certain neurodevelopmental, reproductive and urogenital disorders.
David Kristensen and colleagues conducted a review of experimental animal and cell-based research, and human epidemiological research related to paracetamol use during pregnancy published between 01 January 1995 and 25 October 2020. The authors summarise the research, reporting that prenatal paracetamol exposure in humans might be associated with adverse neurological, urogenital and reproductive outcomes in males and females. These epidemiological findings are supported by experimental studies showing adverse effects in animal and cellular models.
Based on their review and taking a precautionary approach, the authors propose that people should be counselled early on in pregnancy to forego the use of paracetamol unless medically indicated; consult with their physician or pharmacist if uncertain whether to use paracetamol and before using on a long-term basis; and minimise risk by using the lowest effective dose for the shortest possible time. While these recommendations may not differ substantially from current general pregnancy medication advice, the authors believe that paracetamol-specific risk communication is warranted to both health professionals and those who are pregnant because of the high rates of use and perceptions of negligible risk.
Kristensen and co-authors call for agencies such as the US Food and Drug Administration and the European Medicines Agency and appropriate societies to review all available data covering both epidemiological and experimental studies, so that an evidence-based evaluation of the risk can be made available to inform patients and healthcare professionals. They also make some recommendations for the design of robust future human epidemiological studies to better understand the effects of paracetamol exposure during pregnancy.

