 Researchers in the US and Japan have discovered a new mechanism which leads to the joint deterioration associated with osteoarthritis.
Researchers in the US and Japan have discovered a new mechanism which leads to the joint deterioration associated with osteoarthritis.
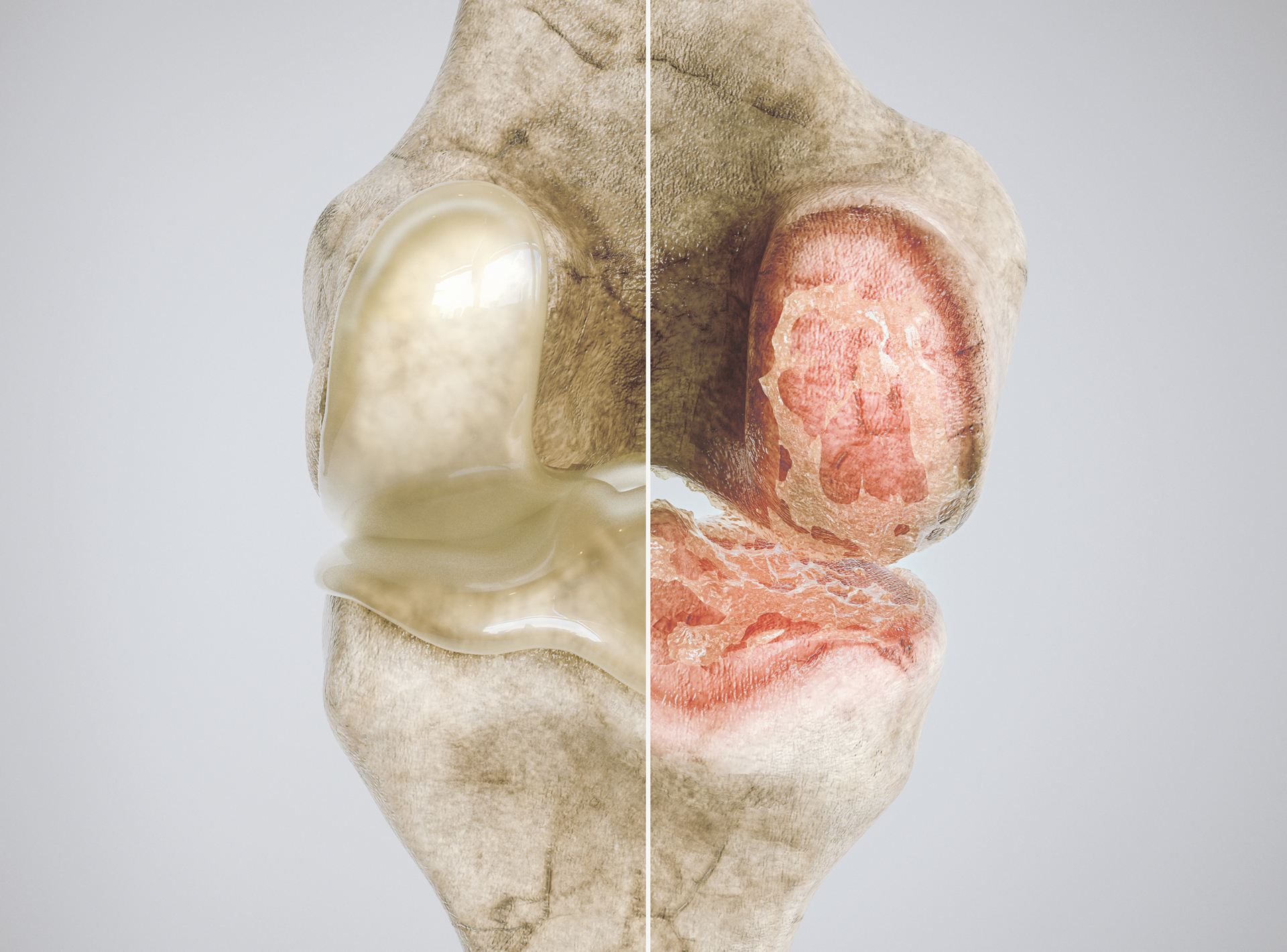
Osteoarthritis is the most common form of arthritis, affecting some 2.2 million Australians, and occurs when cartilage in a joint stiffens and begins to break down, eventually damaging the underlying bone and resulting in pain, swelling and feelings of stiffness.
Much has remained unknown about the molecular causes of this damage and how to treat it, especially in the knees, where no single event causes the cartilage damage, and the greatest predictive risk factor is time – with cartilage stiffness increasing by 2–3 fold with aging.
The study, published 10 January 2023 in Nature Communications, found that increased stiffening of the extracellular matrix (ECM), a network of proteins and other molecules that surround and support tissues in the body, led to a decrease in α-Klotho – a protein linked to longevity – in the cartilage of the knee – causing further damage to the chondrocytes in the healthy cartilage.
Lead author Dr Fabrisia Ambrosio, director of the Atlantic Charter Discovery Centre for Musculoskeletal Recovery of the Schoen Adams Research and Member of the Faculty of Physical Medicine and Rehabilitation at Harvard Medical School, explained that as people age, their α-Klotho levels fall.
“Which is why it is referred to as a longevity protein,” Dr Ambrosio said.
“Yet, while age-related declines in α-Klotho have been linked to the onset of an aged tissue phenotype in many organ systems, our understanding of the molecular mechanisms driving these declines is lacking.
“However, it is well known that mechanical forces induce modulation of nuclear chromatin structures and epigenetic landscapes to impact gene transcription, and it is well established that increased matrix stiffness disrupts chondrocyte functionality via mechanotransducive pathways, ultimately leading to cellular senescence.
“Therefore, we examined whether a loss of α-Klotho in response to stiff substrates was driven by epigenetic modifications.”
Using advanced mass spectrometry technology, the researchers mapped out the trajectory of structural and protein changes in mice with knee osteoarthritis over the course of their lifetimes and according to sex, before comparing their findings to the current understanding of the condition in humans.
“We identified matrix stiffness as a potent epigenetic regulator of α-Klotho,” Dr Ambrosio said.
“The ECM plays a dynamic role in regulating cartilage homeostasis and undergoes extensive remodelling with increasing age.
“Specifically, increased matrix stiffness increased Klotho promoter methylation, downregulated Klotho gene expression, and drove young chondrocytes towards an aged phenotype in vitro, and this age-related decrease in α-Klotho expression was also confirmed in human cartilage.”
Previous studies have shown that α-Klotho protects mitochondria within skeletal muscle and plays a key role in skeletal muscle regeneration following injury and significantly, the team found that exposing aged chondrocytes to a softer extracellular matrix restored the knee cartilage to a more youthful state.
They verified this in models of young and old chondrocyte cells responsible for cartilage formation, which were seeded in environments designed to mimic young and old tissue stiffness.
Young chondrocyte cells looked old when put on a stiff surface due to the loss of α-Klotho, but when the researchers protected the cells from the stiffness in their environment, they chondrocytes remained healthy.
As current interventions for osteoarthritis, such as exercise, weight loss, physical therapy, medications, injections, and joint replacement surgery, are only aimed at reducing pain and improving mobility, the team said their results offered important potential new treatment targets to restore cartilage health.
“This research enhances our mechanistic understanding of why osteoarthritis happens in the first place, and it paves the way for the development of therapeutics to prevent these changes,” Dr Ambrosio said.
“Together, these findings suggest that preventing or reversing age-related matrix stiffening and the resulting pathogenic mechanotransducive signalling may serve as a promising therapeutic target to attenuate or even reverse the development of age-related KOA.
“Such therapeutics are important because there are currently no disease-modifying treatments for osteoarthritis; the best we can do for now is minimize pain and disability.”
The researchers also noted that their results may be applicable to the toll that epigenetic factors caused by aging takes on other tissues throughout the body.
“Since matrix stiffening is a feature of aged tissues throughout the body, we anticipate that these findings may also have implications beyond cartilage repair for the field of aging research,” Dr Ambrosio said.
“We are interested in evaluating whether epigenetic regulation of α-Klotho and other longevity factors by the extracellular matrix may help explain functional decline of tissues throughout the system.”

