The mythological fountain of youth may be one step closer, with the results of a 13-year study from Harvard Medical finding that while the loss of epigenetic information can drive ageing, restoration could reverse it.
The research, published 12 January 2023 in Cell, demonstrated that chemical and structural changes to chromatin, the complex of DNA and proteins that forms chromosomes, fuelled ageing without altering the genetic code itself – and that restoring the integrity of the epigenome reversed those signs of ageing.
Senior author, professor of genetics at Harvard Medical’s Blavatnik Institute, and co-director of the Paul F. Glenn Centre for Biology of Ageing Research, Professor David Sinclair, explained that the discovery supports the hypothesis that mammalian cells maintain a backup copy of epigenetic data that can enable an aged cell to reboot into a youthful, healthy state.
“It is like rebooting a malfunctioning computer,” Professor Sinclair said, speaking with Harvard Research on 12 January 2023.
“[And] it is a permanent reset.
“We hope these results are seen as a turning point in our ability to control aging: this is the first study showing that we can have precise control of the biological age of a complex animal – that we can drive it forwards and backwards at will.”
Historically, the DNA damage most consistently linked to ageing was the double-stranded DNA break (DSB), caused by every-day exposure to simple background radiation such as sunlight – or just breathing – which occur at a rate of 10 to 50 per cell, per day.
The gradual accumulation of DSBs results in deleterious mutations that eventually lead to a loss of cellular function, tissue breakdown and death aging – and a recent comparison of 18 different rodent species showed that, of all DNA repair processes, the repair of DSBs was the most highly correlated with lifespan.
However, recent experiments to test the importance of mutations as a driver of aging have led to questions about DSB’s significance in the overall process.
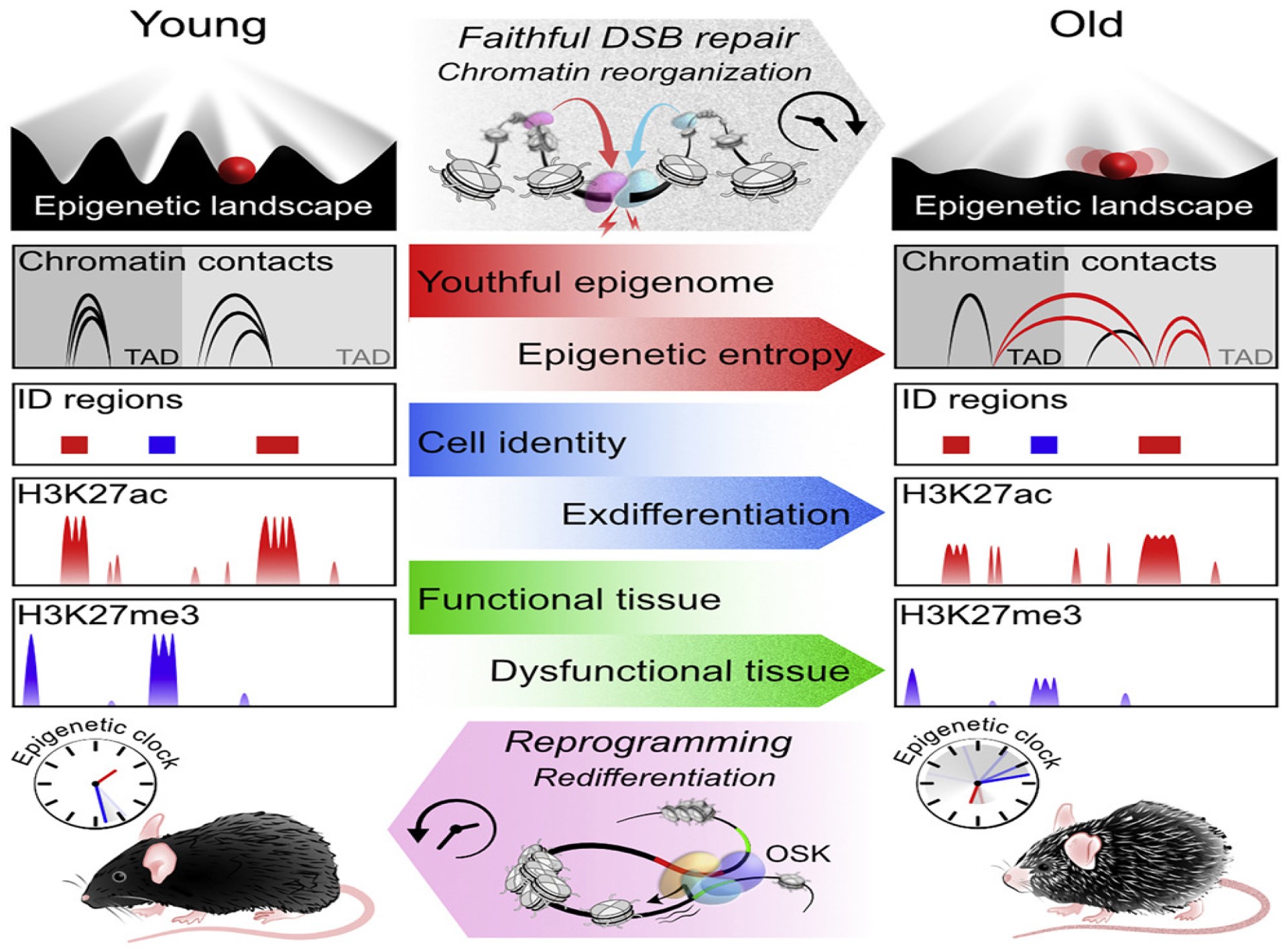
Professor Sinclair explained that to maintain their function, cells utilize energy from their environment to preserve both genetic information encoded in DNA, and ‘epigenetic’ information encoded in transcriptional networks, histone modifications, chromatin conformation, and DNA methylation patterns.
The team’s work back in the early 2,000’s had shown that epigenetic information was also lost over time, but the reasons for that loss, and whether it was a cause or a consequence of ageing, were not known.
“One of the most striking discoveries about the epigenome in recent years is that the methylation status of specific sites changes with predictability during ageing, and can therefore be used to estimate biological age, not just within members of a species but across diverse species, implying a conserved mechanism,” Professor Sinclair said.
“Together, these findings have led to a shift from viewing ageing as a random process to one that is non-random and potentially driven by reproducible and predictable epigenetic changes.
“But key questions remained: what causes the epigenome to change over time, does this cause ageing, and if DNA repair alters the epigenome, how do these seemingly random events produce similar gene expression changes in cells and individuals, making it appear as though it were a program?”
To gain further insights, the team tested whether the altered epigenome of old cells could be recovered while maintaining cell identity, ruling out mutations as a cause of ageing and providing compelling evidence that epigenetic alterations were a cause.
Their methodology involved creating fast-healing DSBs in lab mice, using a transgenic mouse system called ICE – Inducible Changes to the Epigenome – increasing the frequency of the DSBs to simulate a much faster ageing process, giving the ICE mice a lifespan closer to six months rather than 2.5 years.
“Traditionally, the process of DNA damage checkpoint activation and DNA repair has been studied using mutagens or radiation doses that cause DNA damage substantially above background levels,” Professor Sinclair explained.
“The ICE system allows us to create DSBs at levels at more natural levels, only a few-fold above background, thus avoiding overt DNA damage signalling response, cell cycle arrest, aneuploidy, mutations, or cellular senescence.”
Initially, no profound changes at the physiological or molecular levels were observed in the treated ICE mice.
“Over the course of the next 10 months, however, every tissue we examined had deteriorated and developed signs of ageing, and this observation suggests that molecular changes occurring during or shortly after the treatment trigger an advancement of the epigenetic clock many months later,” the team reported.
“We believe ours is the first study to show epigenetic change as a primary driver of ageing in mammals… this is the first evidence that faithful DNA repair alters multiple layers of epigenetic information, including spatial chromatin contacts, chromatin insulation, and P-E communication.
“We regard these results as strong evidence that epigenetic drift, driven by the DSB repair response are a universal cause of ageing in eukaryotes.”
Next, they attempted to reverse the changes they had caused through epigenetic reprogramming, specifically the ectopic expression of the Yamanaka factors Oct4, Sox2, Klf4 and Myc, which are known to induce somatic cell reprogramming, a group dubbed OSK by the researchers.
According to Professor Sinclair, introducing OSK “set in motion an epigenetic program that led cells to restore the epigenetic information they had when they were young,” restoring the mRNA levels of 86% of genes in retinal ganglion cells, for example, that were altered by ageing.
Though the specific mechanism by which OSK achieved this reboot is yet to be understood, Professor Sinclair explained that because it is easier to manipulate the molecules that control epigenetic processes than to reverse DNA mutations, the study shows that epigenetics should be a key focus for the prevention or treatment age-related damage.
“The data strongly argue that the process of DSB repair, even if it doesn’t lead to a mutation, alters the epigenome and accelerates aging at physiological, histological, and molecular levels, including an acceleration of the epigenetic clock, and that it can be restored by epigenetic reprogramming,” he said.
Co-author and research fellow in genetics in the Sinclair lab, Jae-Hyun Yang, who spent 10 years working on the project, believed that their findings would “transform the way we view the process of ageing and the way we approach the treatment of diseases associated with ageing.”
“There are other ways to manipulate the epigenome, like drugs and small molecule chemicals that induce gentle stress, and this work opens a door for applying those other methods to rejuvenate cells and tissues,” Mr Yang said.
Professor Sinclair also hoped their work inspired other scientists to study how to control ageing to prevent and eliminate age-related diseases and conditions in humans, such as cardiovascular disease, type 2 diabetes, neurodegeneration, and frailty.
“These are all manifestations of ageing that we’ve been trying to treat with medicines when they arise, which is almost too late,” he said, noting that the goal would be to address the root causes of ageing to extend the number of years that a person remains not just alive but well.
“We are talking about taking someone who is old or sick and making their whole body or a specific organ young again, so the disease goes away. It is a big idea – it is not how we typically do medicine.”
Medical applications are a long way off and will take extensive experiments in multiple cell and animal models, but studies in nonhuman primates are currently underway and eventually, one day in humans.

