 The patient presents to the emergency department with a 2-week history of intermittent lower abdominal pain, loose stools, myalgia, and low-grade fever.
The patient presents to the emergency department with a 2-week history of intermittent lower abdominal pain, loose stools, myalgia, and low-grade fever.
Background
A 28-year-old man with a medical history significant for asthma presents to the emergency department with a 2-week history of intermittent lower abdominal pain, loose stools, myalgia, and low-grade fever. His symptoms began after an episode of diarrhea and vomiting, which had also affected his wife and children at the same time. Although his family’s symptoms resolved shortly after their onset, his symptoms have progressed. He has been generally active, playing soccer at least twice per week, but he has recently felt too ill to participate in any physical activity.
The patient’s only medication is a salbutamol inhaler, which he uses intermittently for asthma exacerbation. He is employed as a groundskeeper, which involves the occasional handling of raw animal manure. Because of his occupation and manure exposure, his family healthcare provider had considered leptospirosis as a potential diagnosis accounting for his symptoms; however, laboratory examination of the patient’s blood and urine for leptospirosis is negative.
Physical Examination and Workup
Upon physical examination, the patient appears mildly dehydrated, with sunken eyes and decreased skin turgor. Vital signs demonstrate an oral temperature of 100.6ºF (38.11ºC), pulse of 90 beats/min, blood pressure of 121/65 mm Hg, respiration rate of 20 breaths/min, and oxygen saturation of 98% while breathing room air. The abdomen is soft and nondistended, and active bowel sounds are present. Significant tenderness to palpation is noted in the lower abdomen; it is most prominent in the left iliac fossa and suprapubic regions, where localized rebound and guarding are present. No organomegaly or hernias are noted. The remainder of the physical examination findings, including cardiac, respiratory, and neurologic examination, are normal.
A peripheral intravenous line is placed, and blood is drawn and sent for laboratory testing. Abdominal and upright chest radiographs are obtained. Laboratory tests are significant for a white blood cell count of 15.0 × 103 cells/μL (reference range, 3.5-12.5 × 103 cells/μL) and a C-reactive protein level of 212 mg/L (reference range, 0.08-3.1 mg/L). The rest of his laboratory test results are within normal limits, and both the abdominal and chest radiographic examinations are normal.
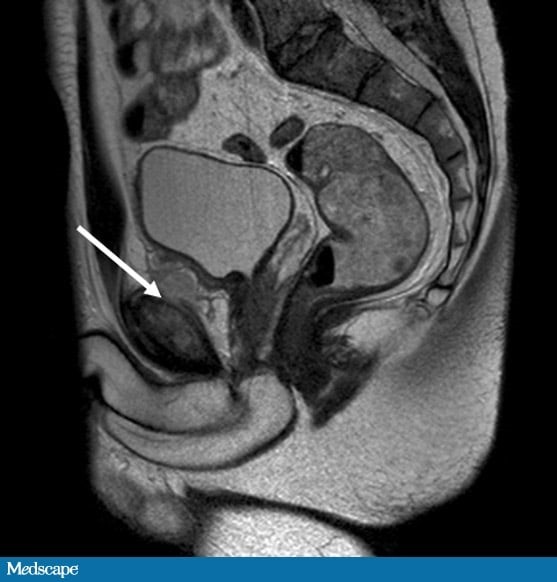
Urine dipstick testing does not demonstrate any blood or leukocyte esterase. CT of the abdomen and pelvis is performed, which demonstrates an area of inflammation deep to the pubic symphysis. MRI of the pelvis is obtained (Figure).
At this time, blood cultures obtained on admission return positive for Staphylococcus aureus.
Based only on these findings, which of the following is the most likely diagnosis?
- Perirectal abscess
- Ruptured diverticulum of the urinary bladder
- Primary sarcoma of the pubic tubercle
- Osteomyelitis pubis
And the answer is: Osteomyelitis pubis.
Your Peers Chose:
- Perirectal abscess 41%
- Ruptured diverticulum of the urinary bladder 19%
- Primary sarcoma of the pubic tubercle 6%
- Osteomyelitis pubis 34% <—- CORRECT!
Breakdown
The MRI (Figure) revealed abnormal marrow signal in the pubis, with periosteal elevation and marked soft-tissue reaction. These features demonstrated a significant inflammatory condition of the pubic symphysis. These MRI findings, along with the proven bacteremia and elevated inflammatory markers, were consistent with a final diagnosis of osteomyelitis pubis.
Inflammation of the fibrocartilaginous pubic symphysis joint is rare and occurs in two forms: infective and noninfective. The noninfective variant, osteitis pubis, was first described by Beer[1] in 1924; it is a self-limited inflammatory condition of the joint and its surroundings. In contrast, osteomyelitis pubis involves infective inflammation of bone, and it accounts for 2% of all reported cases of hematogenous osteomyelitis.[2] Both conditions share a very similar clinical presentation, and distinguishing between them can be difficult.
The etiology of both osteitis pubis and osteomyelitis pubis is not fully understood; similar causative factors have been cited for both conditions. These factors include athletic overexertion, pregnancy and childbirth, urologic or gynecologic manipulation, intravenous drug abuse, and surgery.[3,4] Although the mechanisms by which surgery, childbirth, or intravenous injection result in osteomyelitis pubis can be readily explained by hematogenous dissemination or extension of local infection, athletic exertion as a cause is less straightforward. Certain sports are known to predispose athletes to injury of the groin and pubic symphysis, particularly those that involve repetitive twisting or turning motions at the pelvis, such as soccer, hockey, rugby, and tennis.[4]
One theory is that some form of low-grade localized trauma occurs in the region (which may go unnoticed by the patient), followed by a transient bacteremia opportunistically seeding the damaged area.[5] This transient bacteremia may arise from any number of innocuous causes, ranging from minor skin trauma to dental extraction.[6,7]
The most common pathogen found in patients with osteomyelitis pubis is S aureus, although in intravenous drug users it is more commonly Pseudomonas aeruginosa. In postsurgical cases, mixed gram-negative bacteria are often the causative agents.[7,8,9] Individual cases have also been reported with a wide range of other organisms, such as Streptococcus viridans, Staphylococcus epidermidis, and Salmonella species.
In both osteomyelitis pubis and osteitis pubis, patients usually present with vague unilateral or bilateral pelvic, groin, or lower abdominal pain. The pain generally worsens with exercise, and patients may report difficulty ambulating. When standing or walking, patients tend to lean forward secondary to adductor or rectus muscle spasm. Upon examination, abduction of the hip results in significant pain, and the patient’s range of movement may be diminished as well.
The insidious onset and nonspecific nature of these symptoms, coupled with the unfamiliarity of clinicians with these conditions, leads to a high rate of delayed diagnosis. These entities are often misdiagnosed as subclinical inguinal hernias, coxarthrosis, and adductor muscle spasms.[3] One review of 18 cases of osteomyelitis pubis reported an average delay of 13 days from the onset of symptoms to diagnosis (range, 1-30 days).[7]
In osteomyelitis pubis, symptoms tend to progressively worsen, whereas osteitis pubis is largely self-limited. The key differentiating factor between these conditions is the establishment of (or absence of) infection, which is implicated by signs of systemic infection (such as fever, tachycardia, vomiting, and elevated inflammatory markers) but is only confirmed by verification of the presence of organisms either in blood cultures (in severe cases) or by aspiration or biopsy of the pubic symphysis region.
Plain radiographs are of limited value in the initial stages of both conditions because radiographic changes occur weeks later. In the early stages of disease, MRI is much more sensitive; both conditions will produce some edema of the bone marrow, but the presence of fluid and extensive soft-tissue reaction raises suspicion for osteomyelitis.[4] Three-phase bone scintigraphy normally shows increased uptake in all three phases in osteomyelitis pubis, but uptake is increased only in the mineralization (or delayed) phase in the case of osteitis pubis.[3]
The symptoms and signs of established osteomyelitis pubis (lower abdominal pain, fever, vomiting, tachycardia, and elevated inflammatory markers) closely mimic those of more common lower abdominal pathologies, such as appendicitis or diverticulitis; as such, the rates of negative laparotomy and laparoscopy in these patients are fairly high.
The treatment of osteitis pubis is largely symptomatic, because most cases resolve spontaneously with physical therapy and anti-inflammatory medications. Some physicians advocate more invasive measures, such as injection of steroids or local anesthetics into the joint, or even the use of dextrose prolotherapy (injection of an otherwise nonpharmacologic irritant solution into the region of tendons or ligaments in an attempt to strengthen weakened connective tissue and thus alleviate musculoskeletal pain). No randomized controlled trials of any of these practices have been performed, and a systematic review found only level 4 evidence for all of these therapies.[10]
The mainstay of treatment of osteomyelitis pubis is a prolonged course of antibiotic therapy (initially intravenous) targeted at the causative agent. As many as 50% of cases do not fully resolve with antibiotic therapy alone and may require formal surgical debridement of the area.[9] This debridement involves curettage and jet lavage; some surgeons also implant antibiotic-impregnated beads into the affected area.[3] Whether or not surgery is performed, targeted antibiotic therapy is recommended until the erythrocyte sedimentation rate has normalized, which generally requires at least 6 weeks of antibiotic therapy.[4]
In this case, antibiotic therapy was changed to full-dose intravenous flucloxacillin based on the microbiologic sensitivities of the organisms recovered from the blood cultures. The patient was given nonsteroidal anti-inflammatory medication for pain control. Within 36 hours of this targeted antibiotic therapy, his pain improved and he remained afebrile. After daily physical therapy, he was discharged to home on hospital day 9, ambulating with crutches, and he was instructed to continue oral flucloxacillin for 8 weeks postdischarge. After completion of his antibiotic course, his symptoms had entirely resolved; he had no residual disability and was back to playing soccer.
More details
- Original story: A 28-Year-Old Soccer Player With Odd Abdominal Pain, Fatigue (medscape.com)
- Authors: Thomas D. Pinkney, MB ChB; Simon F. Hobbs, MB ChB; Timothy D. Stone, MB ChB; Tim C.F. Sykes, MD, BSc, MB BCh, FRCS
- All content for this article belongs to Medscape.com. For more case examples, visit: Case Challenges – Index (medscape.com)

