Perth-based biotech company Emyria has obtained approval for a Mobile phone app that will help clinicians monitor the heart of patients.
Perth-based biotech company Emyria has obtained approval for a Mobile phone app that will help clinicians monitor the heart of patients.
Earlier this week, on May 12, the Therapeutic Goods Administration (TGA) approved Australia’s first Class IIa “software-as-a-medical-device” (SaMD) for monitoring heart health.
Class IIa classification applies to any software that handles information used to make decisions about diagnosis or treatment. Successful classifications under this scheme require extensive verification and clinical evaluation procedures and a higher level of scrutiny by the TGA.
The mobile app, called Openly, runs on Apple and Android smartphones and will allow clinicians to monitor heart rate, heart rate variability, and atrial fibrillation.
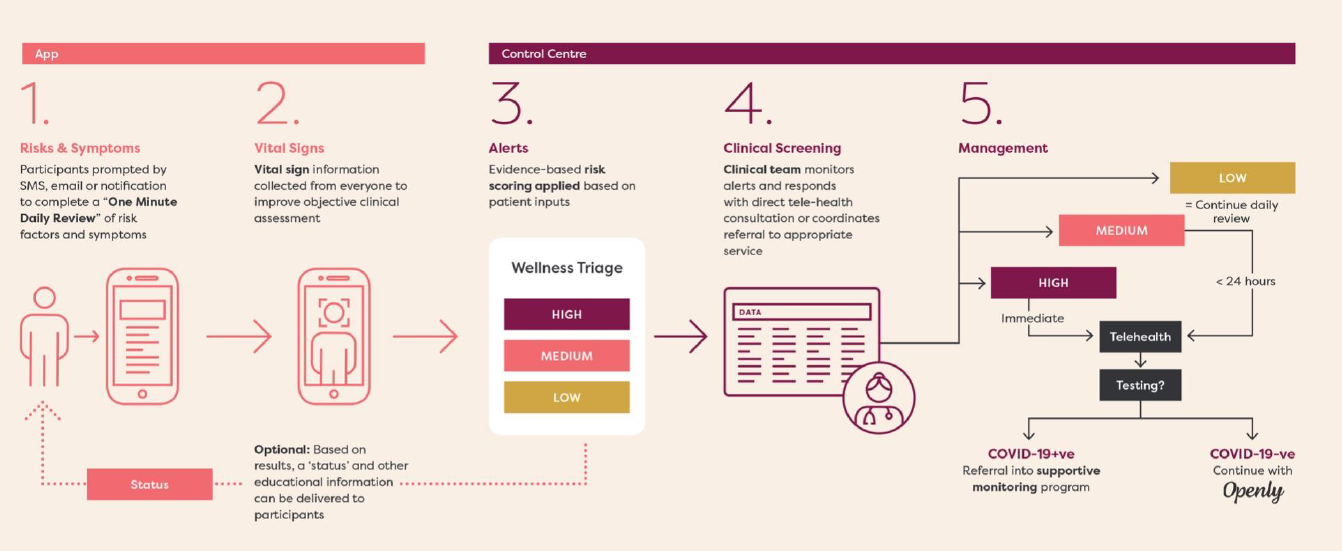
Openly, officially called “Smartphone camera home cardiovascular monitoring application software”, is the first of its kind in Australia and represents an important milestone in Emyria’s goals to remotely capture health data for various drug development, telemedicine and consumer healthcare projects.
“Obtaining this classification highlights Emyria’s commitment to formal regulatory approval of novel drug treatments and technologies for unmet clinical needs,” said Emyria’s Managing Director, Dr Michael Winlo in an announcement.
“Class IIa registration means Emyria’s Real-World Evidence platforms are now capable of capturing medical grade vital signs using just an Apple or Android smartphone. Emyria plans on using this capability in our upcoming drug development programs which allows our clinical teams to remotely monitor additional safety and efficacy outcomes data in our trial participants,” Dr Winlo added.
How it will be used
The Openly app will initially function as a tool to capture clinical data remotely from patients taking part of upcoming research projects led by Emyria:
- EMD-003 – “pursuing a Schedule 3 registration of a cannabinoid-based medicine targeting psychological distress”;
- EMDMA-001 – “An MDMA-assisted psychotherapy trial for post-traumatic stress disorder (PTSD)” and;
- EDICT – “An advanced digital monitoring and engagement platform for at-risk and confirmed COVID-19 individuals”.
In the future, Openly may also help with the remote monitoring of cardiac patients. “We also believe this capability has applications in a variety of medical and consumer health monitoring settings here medical-grade remote monitoring can improve the care of patients with complex needs,” Dr Winlo said.