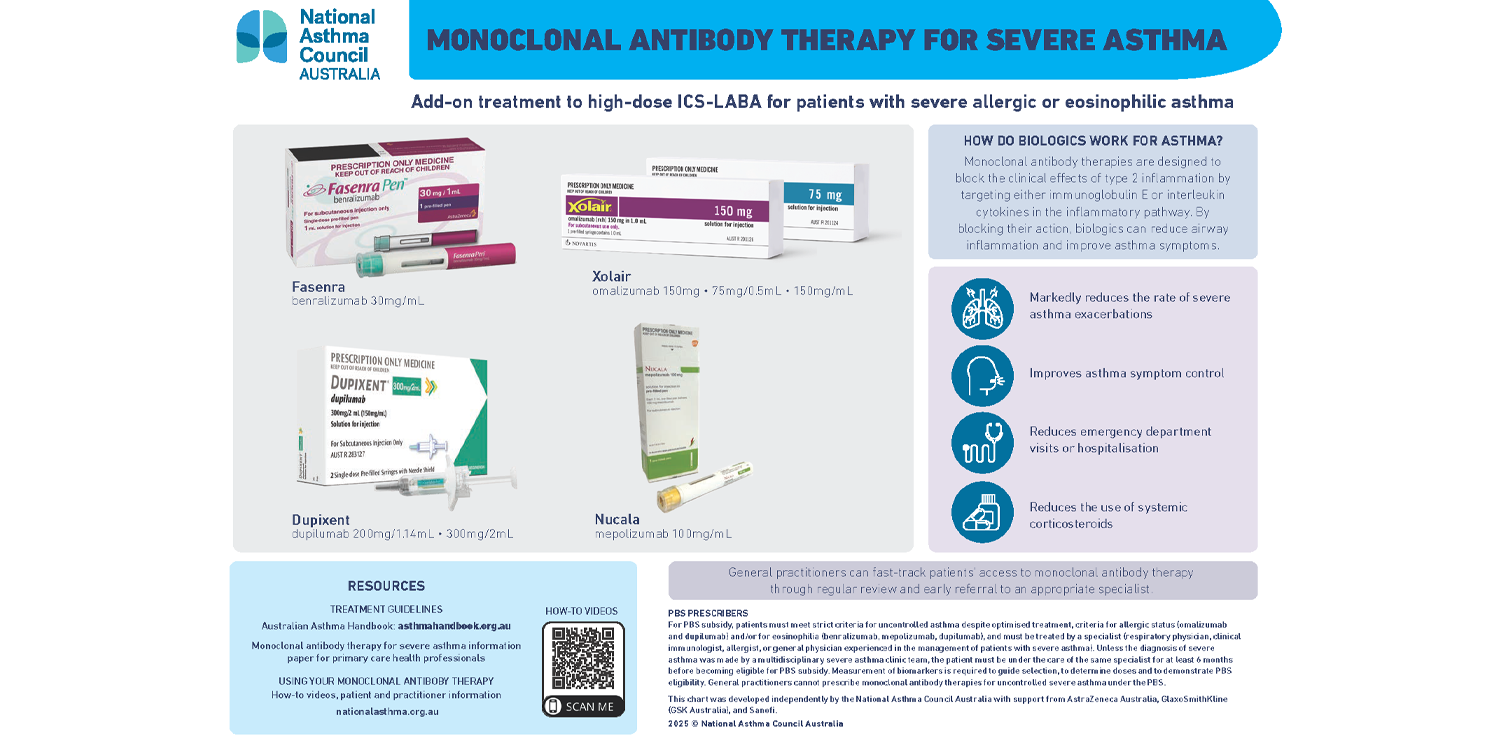
An updated information paper and wall chart on monoclonal antibody therapy for severe asthma has been released to assist primary health practitioners.
The resources, compiled by the National Asthma Council Australia, were designed to assist GPs to fast-track patients’ access to monoclonal antibody therapy through regular review and early referral to an appropriate specialist.
Clinical Associate Professor and Clinical Executive Lead at the Council Debbie Rigby said the updated information paper and wall chart provide “a clear and concise framework” to navigate what can be a complex clinical area.
RELATED: Asthma surges in the outer suburbs
“Monoclonal antibody therapy is the most effective add-on treatment for patients with severe allergic or eosinophilic asthma that does not respond adequately to treatment with high-dose inhaled corticosteroids (ICS) in combination with long-acting beta2 agonists (LABAs),” she said.
“These biologic therapies for severe asthma are designed to block the clinical effects of type 2 inflammation by targeting either immunoglobulin E or interleukin cytokines in the inflammatory pathway.
“By blocking their action, biologics can markedly reduce the rate of severe asthma exacerbations, improve symptom control and reduce the use of systemic corticosteroids in patients with severe asthma and persistent type 2 airway inflammation.”
RELATED: Asthma chart updated
The newly released chart is a quick reference tool that lists the four available biologic treatments currently available in Australia, which are benralizumab, omalizumab, dupilumab and mepolizumab.
A clinical trial at King’s College London, supported by researchers in WA, showed benralizumab, traditionally used in the management of severe asthma, could be re-purposed in emergency settings to reduce the need for further treatment and hospitalisations.
The trial saw the drug injected via a single dose at the point of exacerbation.
Lead investigator of the trial Professor Mona Bafadhel from King’s Centre for Lung Health said this could be “a game-changer” for people with asthma and chronic obstructive pulmonary disease (COPD).
RELATED: New COPD guideline for doctors
The NAC’s updated information paper includes key practice points, information about use in pregnancy and ongoing care of patients receiving monoclonal antibody therapy.
“This includes advising patients using monoclonal antibody therapy that they must keep taking maintenance ICS-LABA treatment and ensuing they have an up-to-date written asthma action plan to follow when symptoms worsen,” Associate Professor Rigby added.
She said the monoclonal antibody therapies currently available in Australia for severe asthma treatment were “generally well tolerated and are not associated with clinically significant unwanted immunosuppression”.
While injection site reactions were common, “systemic reactions, including anaphylaxis, are rare”.
RELATED: Look for ‘silent’ asthma patients
Monoclonal antibody therapies are subsidised by the Pharmaceutical Benefits Scheme for patients in specialist care who meet strict criteria.
Further information on severe asthma can be found in the Australian Asthma Handbook.
Want more news, clinicals, features and guest columns delivered straight to you? Subscribe for free to WA’s only independent magazine for medical practitioners.
Want to submit an article? Email [email protected]


