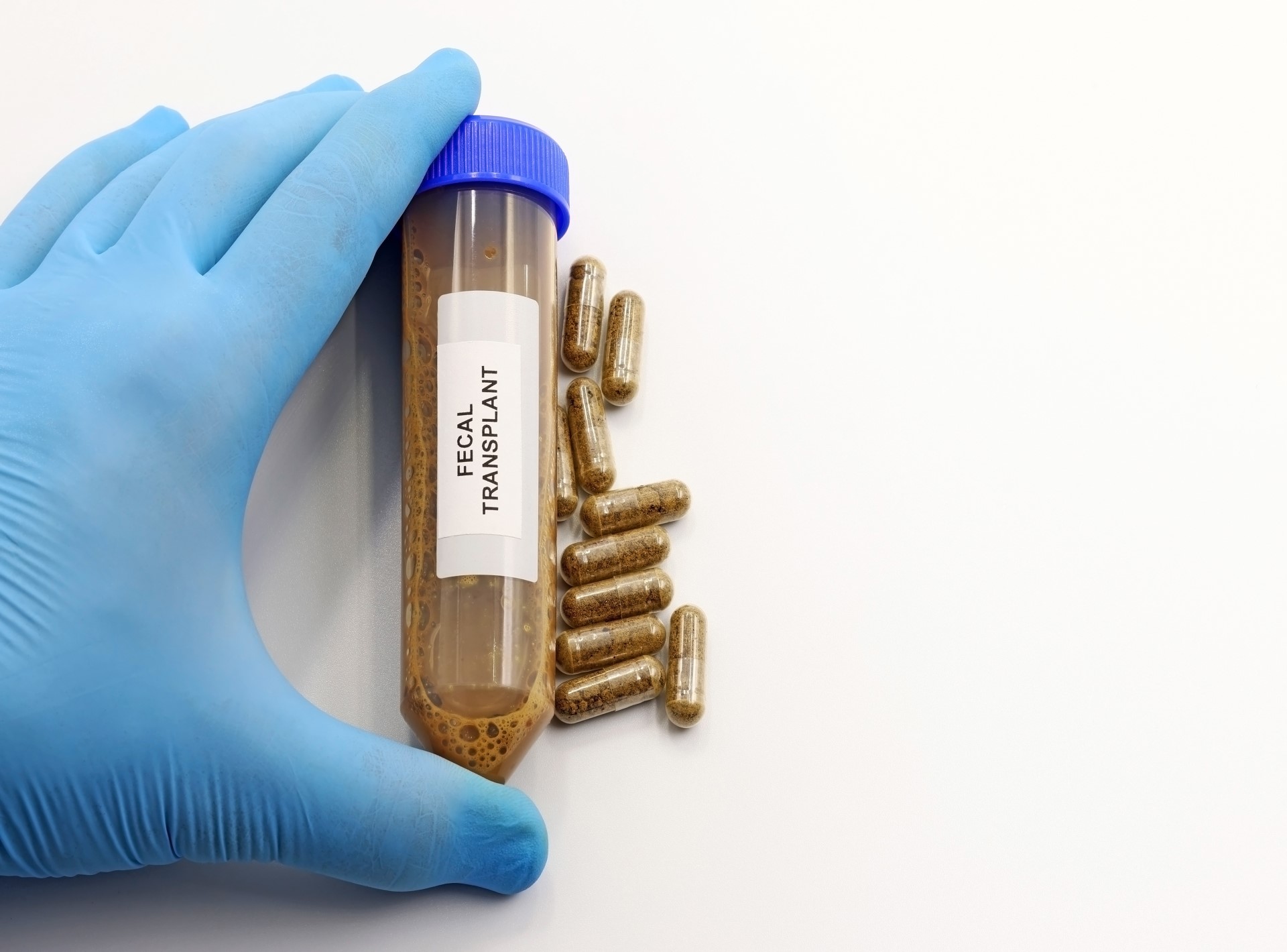
Curtin University’s new poo pills may be coming to a microbiome near you, thanks to a groundbreaking partnership with Australian Red Cross Lifeblood to develop a faecal transplant capsule.
The more patient-friendly, orally administered frozen liquid Faecal Microbiota Transplantation (FMT) capsules could be used in large clinical trials with patients suffering from serious infections of the gastrointestinal tract and to investigate the use of FMT for a range of other medical conditions.
Australian Red Cross Lifeblood successfully submitted a proposal via the WA Government’s Market-led Proposals pathway to expand its FMT program in Perth, with $2.5 million in State funding awarded this year.
FMT has already been demonstrated in studies to be 90% effective in treating the serious bacterial infection, recurrent Clostridioides difficile (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9506672/), which the team described as “pervasive and debilitating,” noting that the most recent data from the US showed that “one in 11 people over the age of 65” diagnosed with the condition died within one month of infection.
As such, Lifeblood Executive Director Stuart Chesneau said there was growing interest internationally in FMT capsules as a simpler delivery method for faecal transplantation.
“There is a pressing demand to make FMT more accessible for clinical trials so we’re very excited to partner with Curtin on a project which has the potential to remove cost and time barriers for patients,” Mr Chesneau said.
Project leads, Dr Hani Al-Salami and Dr Armin Mooranian from the Curtin Medical School and the Curtin Health Innovation Research Institute, emphasised the big potential of the project.
“This venture to develop a new platform for pharmaceutical encapsulation of FMT frozen liquid will be our first step towards delivering a prototype for clinical trials,” Dr Al-Salami said.
“Unlike invasive methods of delivery, such as colonoscopy, these capsules will enable frequent dosing for amore tailored and more effective treatment regimen, offering new hope to patients battling resistant infections which don’t respond to standard therapies.”
Curtin’s Vice-Chancellor, Professor Harlene Hayne, said the collaboration with Lifeblood reflected a shared vision to advance medical innovation and enhance patient outcomes.
“This partnership is an exciting opportunity to leverage our organisations’ research excellence and revolutionise how biologicals are being used to treat life-threatening diseases,” Professor Hayne said.
“The collaboration could see a significant step forward in advancing patient care and accessibility to treatment for thousands of Australians experiencing debilitating gut conditions.”
The collaboration, based at the Rotary WA Health Innovation Centre in Perth, aims to have a prototype liquid FMT capsule ready for clinical trials later this year.

