Biologics have revolutionised the treatment of rheumatoid arthritis and psoriatic arthritis. They are increasingly also being used in dermatology, cancer treatment and even MS.

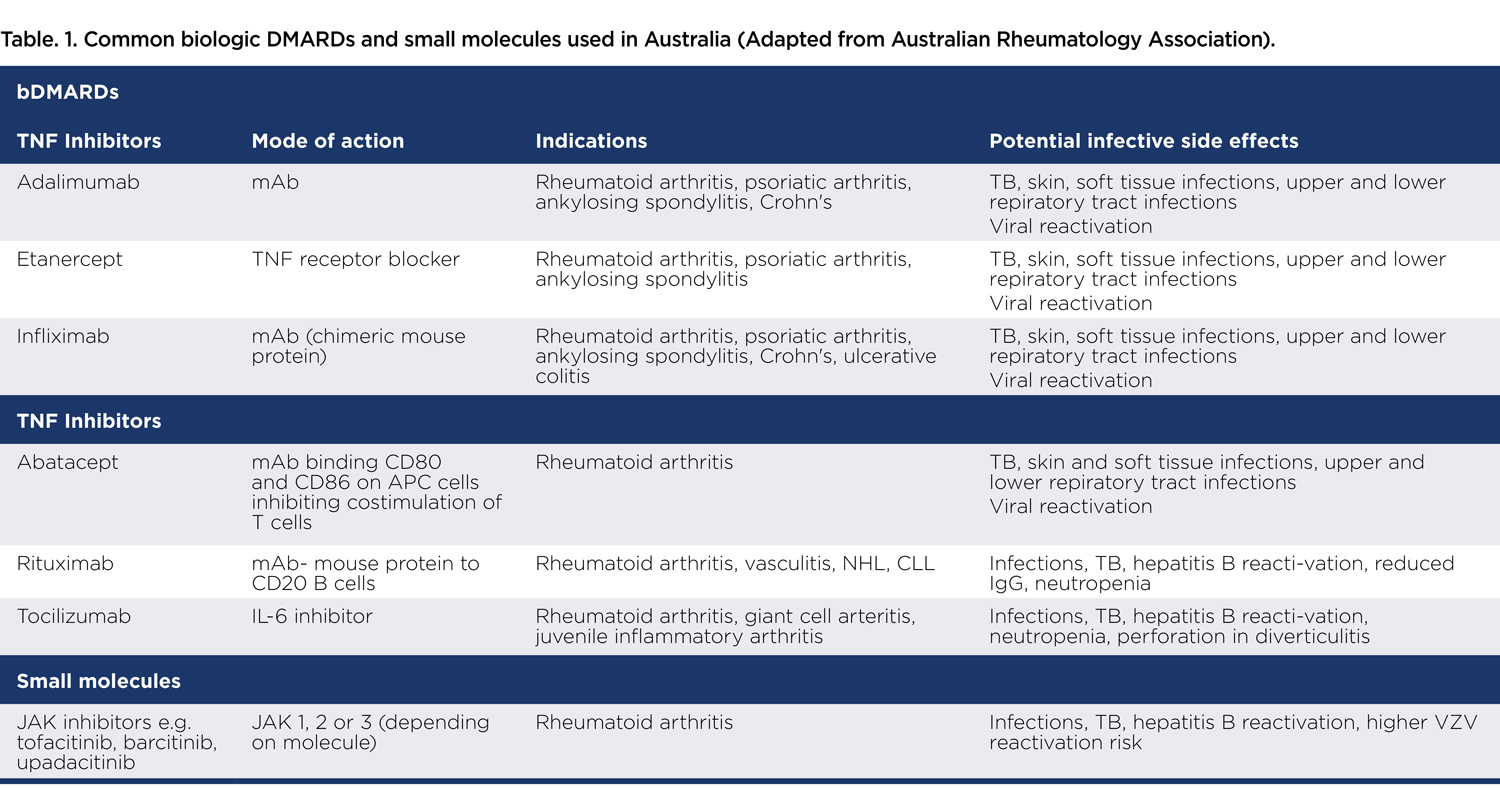
Australians spend more than $2 billion a year on biologics and other targeted therapies. However, they can increase the risk of infection. Particularly in the 12 months after commencement of biologic therapy, upper and lower respiratory tract infections, followed by skin and soft tissue infections are common.
Other infections such as reactivation of tuberculosis, hepatitis B and herpes zoster virus are also well described. TNF inhibitors, such as infliximab and adalimumab have the highest risk, well above that conferred by conventional DMARDs and the autoimmune conditions themselves. The elderly, those with other co-morbidities, particularly diabetes, and those on concomitant steroids are at highest risk.
Predicting infections is difficult because there aren’t any biomarkers that reliably predict infections. However, it is recognised that low lymphocyte counts (<0.5 x10^9/L) and a baseline low CD8+ T-cell (<200 cells/uL) predispose to the development of infections in this population. Among patients on rituximab, a low IgG level (6-7g/L) increases the risk of severe infection.
It is important to note that patients on biologics have a blunted immune response to infection and therefore may not develop a fever but could have hypothermia instead. They may not have a productive cough even if they have pneumonia and radiology reveals multi-lobar involvement. They can also rapidly advance from minor upper respiratory tract viral symptoms to a bacterial lower respiratory tract infection.
They are at risk of common pathogens such as pneumococcus but can also develop fungal and mycobacterial infections. Herpes zoster reactivation can be multi-dermatomal and atypical without the classic vesicular rash. A high index of suspicion for detecting subtle infection presentations is needed.
 Biologics and COVID-19
Biologics and COVID-19
Since the beginning of the pandemic there has been concern and anxiety around continuing the use of biologics in these individuals. Interestingly, several large retrospective studies have shown no excess hospitalisations or increase in the severity of disease or deaths from COVID-19 in this population.
The reason for this is still unknown but one postulate is that biologics modify the cytokine storm due to SARS-CoV-2 infection, thus appearing to have a relatively protective effect.
Biologics have been trialed in patients with an oxygen requirement due to COVID-19. Tocilizumab, an IL-6 inhibitor, has been used in hospitalised COVID-19 patients. However, this is not a recognised treatment, and it is only used within clinical trials. Patients on biologics for their rheumatic and autoimmune conditions should continue these drugs during the pandemic.
Data on preventing infection is lacking and recommendations are based on expert opinion. However, it is widely accepted that commencing treatment for latent tuberculosis infection (with rifampicin for 4 months) in those with positive TST or IGRA tests prior to starting a biologic is important to prevent
TB reactivation.
Patients who are HBsAg-positive or anti-HBc-positive receiving rituximab have the highest risk for hepatitis B reactivation. Antiviral prophylaxis with entecavir or tenofovir is recommended in these cases and should continue for at least 12 to18 months after rituximab is completed to avoid late reactivation.
For patients with anti-HBc-positivity receiving other immunosuppressive therapies and with no evidence of active disease, monitoring with HBV DNA at 3-month intervals is reasonable.
 Often forgotten but an important issue in this population is the risk of infection due to long-term steroids (over six months). Once established on a biologic, corticosteroids, if possible, should be tapered.
Often forgotten but an important issue in this population is the risk of infection due to long-term steroids (over six months). Once established on a biologic, corticosteroids, if possible, should be tapered.
Ensuring childhood vaccines and boosters (particularly pertussis, influenza, and pneumococcal vaccines) are up to date two to four weeks prior to starting a biologic is best. These vaccines, if not previously administered, should be given at any time during a biologic because they confer protection even if their effect is not optimal once the biologic has started.
Live vaccines are contraindicated in patients on biologics and herpes zoster reactivation is common, thus primary VZV vaccination to those not immune or the shingles vaccine, need to be administered prior to the biologic starting. COVID vaccines have been shown to be less effective in this population, but they are still indicated and should be encouraged in all individuals on biologics.
Key messages
- Biologics can increase the risk of infection
- Data does not show increase hospitalisations for Covid-19 in those on biologics
- Prevention depends on individual circumstances.
Author competing interests- nil

